
As for the rightmost Carbon, we also have a tetrahedral where Carbon binds with one Carbon and 3 Hydrogens. Continuing this trend, we have another tetrahedral with single bonds attached to Hydrogen and Carbon atoms. Again, we have 4 electron groups which result in a tetrahedral. This Carbon has 2 single bonds to 2 Carbons and 2 single bonds to 2 Hydrogens. By checking the geometry of molecules chart above, we have a tetrahedral shape.

That means that we have 4 electron groups. We see that C has three single bonds to 2 Hydrogens and one single bond to Carbon. If we break down each Carbon, the central atoms, into pieces, we can determine the relative shape of each section. You can view a better structural formula of butane at en./wiki/File:Butane-2D-flat.png C-C-C-C is the simplified structural formula where the Hydrogens (not shown) are implied to have single bonds to Carbon.
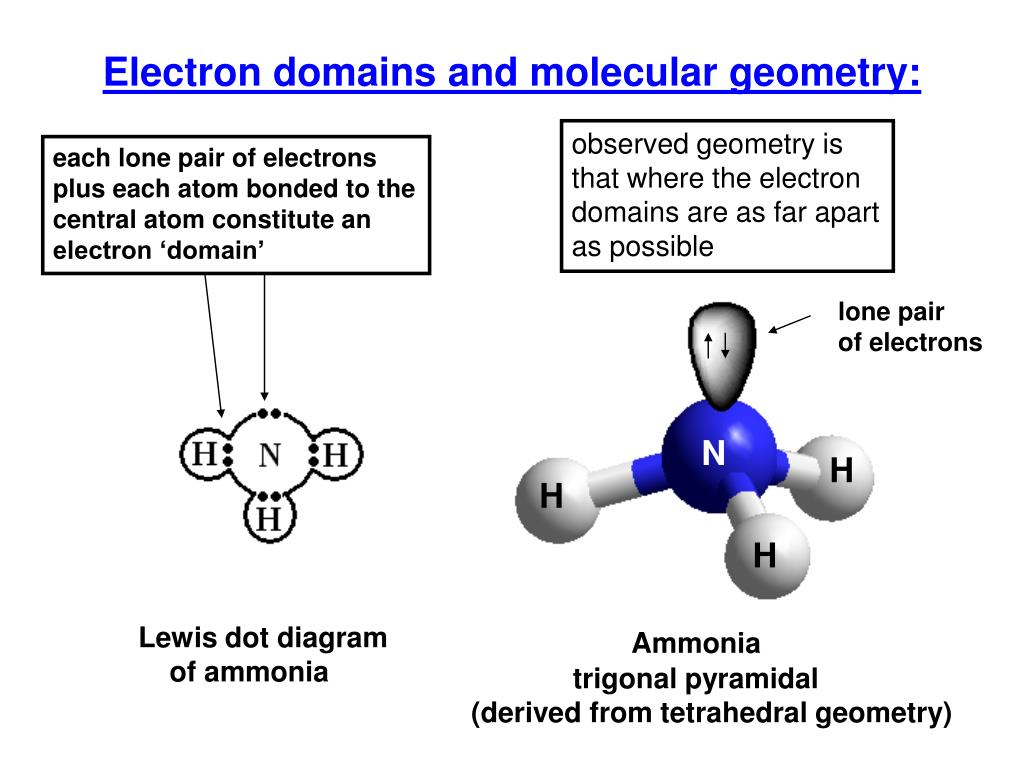
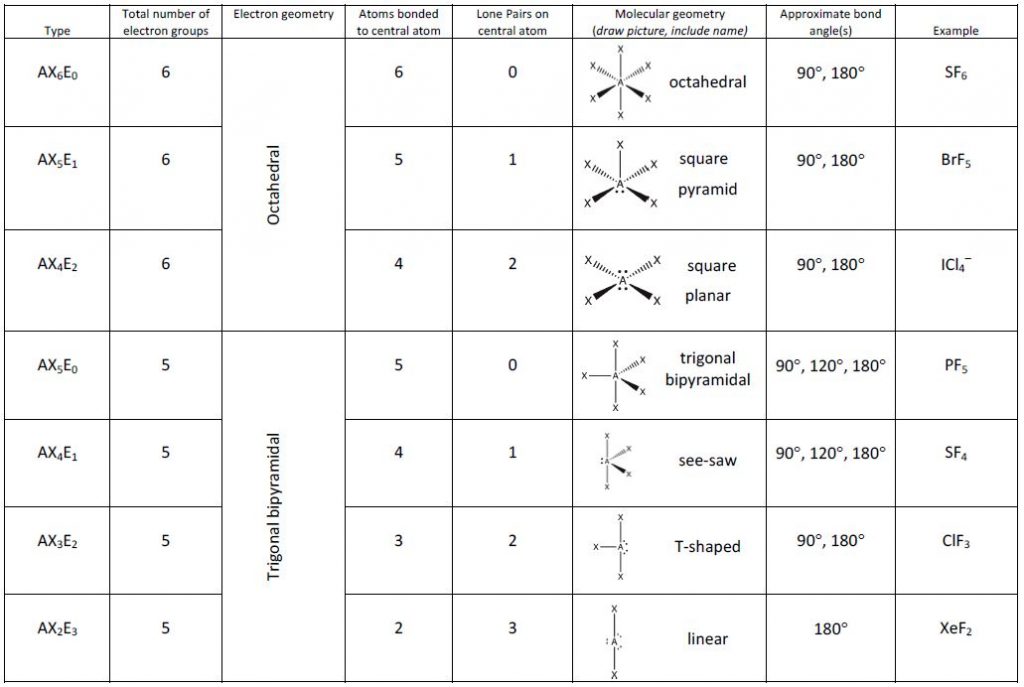
Follow the example provided below:īutane is C 4H 10. In other words, we take long chain molecules and break it down into pieces. For the final description, we combine the separate description of each atom. We take in account the geometric distribution of the terminal atoms around each central atom. The VSEPR theory not only applies to one central atom, but it applies to molecules with more than one central atom. Molecules with More than One Central Atom Geometry of Molecules Chart Number of Electron Groups For example, a molecule with two bond pairs and two lone pairs would have this notation: AX 2E 2. The x represents the number of lone pairs present in the molecule. When lone pairs are present, the letter E x is added. "A" represents the central atom and n represents the number of bonds with the central atom. The VSEPR notation for these molecules are AX n. See the chart below for more information on how they are named depending on the number of lone pairs the molecule has.Īs stated above, molecular geometry and electron-group geometry are the same when there are no lone pairs. When the electron groups are all bond pairs, they are named exactly like the electron-group geometry. Molecular geometry, on the other hand, depends on not only on the number of electron groups, but also on the number of lone pairs. We separate this into two categories, the electron-group geometry and the molecular geometry.Įlectron-group geometry is determined by the number of electron groups. Although VSEPR theory predicts the distribution of the electrons, we have to take in consideration of the actual determinant of the molecular shape. Thus, the molecule's shape reflects its equilibrium state in which it has the lowest possible energy in the system. The electrons and the nuclei settle into positions that minimize repulsion and maximize attraction. The shape of a molecule is determined by the location of the nuclei and its electrons. Using the VSEPR theory, the electron bond pairs and lone pairs on the center atom will help us predict the shape of a molecule. An electron group can be an electron pair, a lone pair, a single unpaired electron, a double bond or a triple bond on the center atom. VSEPR focuses not only on electron pairs, but it also focus on electron groups as a whole. Thus, electron pairs will spread themselves as far from each other as possible to minimize repulsion. The valence-shell electron-pair repulsion (VSEPR) theory states that electron pairs repel each other whether or not they are in bond pairs or in lone pairs. Now that we have a background in the Lewis electron dot structure we can use it to locate the the valence electrons of the center atom. Valence-Shell Electron-Pair Repulsion Theory


 0 kommentar(er)
0 kommentar(er)
